
As clearly stated by the title of the report
"Formaldehyde Derived From Dietary Aspartame Binds To Tissue Components In Vivo"
"Formaldehyde Derived From Dietary Aspartame Binds To Tissue Components In Vivo"
Terug naar de website Summary Discussion AcknowledgementsMaterials & Methods Results References
Aspartame converts to formaldehyde in vivo in the bodies of laboratory rats.
The study by C. Trocho et al, sometimes referred to as the "Barcelona report", or the "Barcelona study", because it was conducted by the staff of the Biology Department of the University of Barcelona, clearly shows that aspartame which was labeled with carbon 14 isotope was transformed into formaldehyde in the bodies of the living specimens and that when they were examined later, the radioactive tagged formaldehyde was spread throughout the vital organs of their bodies. This conclusively proves that aspartame does indeed convert to formaldehyde in the bodies of aspartame consumers, and that many of the symptoms reported by victims of aspartame toxicity are indeed those associated with the poisonous and cumulative effects of formaldehyde.

C. Trocho, R. Pardo, I. Rafecas, J. Virgili, X. Remesar, J.A. Fernández-López and M. Alemany Departament de Bioquimica i Biologia Molecular, Facultat de Biologia, Universitat de Barcelona, 08028
Barcelona Spain. (Received in final form May 13, 1998)
Adult male rats were given an oral dose of 10 mg/kg aspartame 14C- labeled in the methanol carbon. At timed intervals of up to 6 hours, the radioactivity in plasma and several organs was investigated. Most of the radioactivity found (>98 % in plasma, >75 % in liver) was bound to protein. Label present in liver, plasma and kidney was in the range of 1-2 % of total radioactivity administered per gr or mL, changing little with time. Other organs (brown and white adipose tissues, muscle, brain, cornea and retina) contained levels of label in the range of 1/12 to 1/10th of that of liver. In all, the rat retained, 6 hours after administration about 5 % of the label, half of it in the liver. The specific radioactivity of tissue protein, RNA and DNA was quite uniform.
The protein label was concentrated in amino acids, different from methionine, and largely coincident with the result of protein exposure to labeled formaldehyde. DNA radioactivity was essentially in a single different adduct base, different from the normal bases present in DNA.
The nature of the tissue label accumulated was, thus, a direct consequence of formaldehyde binding to tissue structures. The administration of labeled aspartame to a group of cirrhotic rats resulted in comparable label retention by tissue components, which suggests that liver function (or its defect) has little effect on formaldehyde formation from aspartame and binding to biological components. The chronic treatment of a series of rats with 200 mg/kg of non-labeled aspartame during 10 days resulted in the accumulation of even more label when given the radioactive bolus, suggesting that the amount of formaldehyde adducts coming from aspartame in tissue proteins and nucleic acids may be cumulative. It is concluded that aspartame consumption may constitute a hazard because of its contribution to the formation of formaldehyde adducts.
Aspartame is one of the most widely used artificial sweeteners. Its peptide nature: aspartyl-phenylalanine methyl-ester facilitates its intestinal hydrolysis and the absorption (I -3) of innocuous amino acids together with small amounts of free methanol, far away from the lower limits of toxicity for that compound (4). The use of large amounts of aspartame in the diet, however, has been claimed to be the cause of a number of ailments, like headaches (5) and other symptoms (6-7), which are difficult to explain (8) from its known composition and the easy blending of its building components in the overall host metabolism. A number of studies have linked aspartame with neurologic pathologies, but most of the results yielded negative or inconclusive correlation's (9-16). The acute toxicity of aspartame is believed to be low (I7), which has promoted a wide distribution of the product as a potent hypocaloric and safe substitute of sugar (I 8-19).
Methanol is primarily oxidized in several tissues to formaldehyde and formic acid (20-2 1), the latter being considered the main metabolite responsible for the deleterious effects of acute methanol intoxication in man (22), but also in experimental animals (23), in spite of the marked resistance of the rat to formate (24-25). The enzymes involved in methanol metabolism are alcohol dehydrogenase (EC 1. 1. 1. 1) and aldehyde dehydrogenase (EC 1.2.1.3), as well as the microsomal oxidase pathway (26). Acute methanol intoxication may produce blindness and hepatic loss of function (27-28), since the retina, cornea and liver contain the highest alcohol dehydrogenase activity (29-30). These tissues are, thus, where one can expect, eventually, the largest accumulation of their byproducts: formaldehyde and formate, in the event of intoxication. It may be assumed that liver functional failure due to cirrhosis could result in the loss of its role as barrier to intestinal methanol, and thus, the effects of methanol intoxication on other tissues (i.e. the retina) would be more marked. The cirrhotic rat may be, then, used as a model of acute or chronic methanol toxicity.
Formaldehyde is a highly reactive small molecule which strongly binds to proteins (3 1) and nucleic acids (32) forming adducts which are difficult to eliminate through the normal metabolism pathways.
As a result, formaldehyde induces severe functional alterations (33), including the development of cancer (34). The small amounts of formaldehyde which can be potentially produced from dietary use of aspartame have been often overlooked in its potential toxicity precisely because of the limited amount eventually produced. However, the administration of labeled aspartame to experimental animals results in the incorporation of a significant proportion of the label to proteins (35). The accumulation of label has been postulated to be the consequence of label drift into amino acids (essentially in the methionine methyl group) through the one-carbon pool (35). This aspect has not been, however, proved nor further investigated.
We have intended here to determine the extent of conversion of aspartame methanol to formaldehyde and its eventual effect on the overall physiologic function of the rat. In addition we have probed whether the aspartame methanol carbon presence in tissue components is due to the eventual drift of label into methionine and nucleic acid components through the one- carbon pool, or is the consequence of a direct reaction with free formaldehyde forming stable adducts.
The lower incorporation of methanol label in most tissues of cirrhotic rats, compared with controls, may be the consequence of reduced liver uptake of substrates, but also the result of a reduced overall metabolic activity in the damaged liver of the rats (44). These effects are clearly reflected by their stunted growth and high mortality rate during the intoxication process, of about 50 % of the rats (36). The relative insignificance of the differences between the normal and cirrhotic groups indicates that the liver is not essential in the process of transfer of aspartame carbon to tissue proteins, i.e. that there is not a direct relationship between the ability to process alcohols and the retention of methanol carbon, bound to tissue components.
The high label presence in plasma and liver is in agreement with the carriage of the label from the intestine to the liver via the portal vein. The high label levels in kidney and, to a minor extent, in brown adipose tissue and brain are probably a consequence of their high blood flows (45). Even in white adipose tissue, the levels of radioactivity found 6 hours after oral administration were 1/25th those of liver. Cornea and retina, both tissues known to metabolize actively methanol (21,28) showed low levels of retained label. In any case, the binding of methanol-derived carbon to tissue proteins was widespread, affecting all systems, fully reaching even sensitive targets such as the brain and retina.
In all groups studied, the label bound found in plasma and tissues corresponds to that injected with aspartame, since there is no other source of radioactivity available. The lack of changes in plasma radioactivity from 1 to 24 h suggests that the half life of this newly added carbon was quite long, thus precluding the possibility that the label detected would simply correspond to unattached methanol. The label bound to plasma proteins was not aspartame either, since the latter is a non-reactive molecular species fully hydrolyzed in the intestine (1-2); the peptide never arrived to be in contact with the rat tissues or its components. We were not able to reproduce any direct labeling of protein exposed either to aspartame, methanol nor formic acid.
Most of the label found in the tissues is the result of the formation of formaldehyde or (in smaller proportion in any case, because of its lower reactivity) formate adducts. Methanol is highly volatile and, eventually, its radioactivity could hardly be taken into account, since the counting method already eliminates this possibility. In addition, the stabilized maintenance of the plasma radioactivity levels could not belong to formate nor methanol, since these unattached substrates are easily taken up and oxidized by tissues, filtered by the kidney or even lost through respiration as occurs with short chain volatile alcohols. The shape of the time-course graph representing the changes in tissue label supports the hypothesis assuming that the label is firmly bound at least for 6 hours after administration of aspartame. This behaviour is also found in formaldehyde-protein adducts (3 1), long lived species difficult to eliminate, in which the protein is denatured and its original function altered.
The transfer of "one-carbon" units from aspartame to plasma and tissue proteins has been known for a time (35). Its nature, and the mechanism of attachment, however, were assumed to be due to the incorporation of the methanol carbon to normal amino acid structures (essentially forming the methyl group of methionine) through the "one-carbon " tetrahydrofolate and S-adenosyl- methionine pathways (35). The lack of radioactivity in the methionine spot from aspartame treated labeled rat proteins, however, shows that this assumption could no longer be maintained. The finding of other -different-labeled DNP- derivatized amino acid(s) in the exposed protein hydrolysates confirms that the label was not carried into protein through the one-carbon pool metabolism labeling of methionine, i.e. prior to the synthesis of the protein. The coincidence of this labeled DNP-amino acid residue with that obtained from protein experimentally exposed to formaldehyde confirms that the label fixed to rat proteins after labeled aspartame exposure was derived from formaldehyde adducts, and definitely proves that the label in tissue proteins does not correspond to methionine.
This agrees with the incorporation of the label into the fully synthesized protein at a remarkably uniform rate of label distribution between different molecular species in spite of their eventually different turnover (synthesis) rates.
The analysis of label distribution in the nucleic acids shows a remarkable uniformity in the specific activities of DNA and protein, with RNA showing somewhat lower values in the same range. This distribution is in agreement with a fairly uniform exposure to the same reacting species, and could not be explained through incorporation of one-carbon pathways into molecules which show widely different half lives, as is the case with the highly recycled RNA and some proteins and long-lived DNA and proteins. The finding of large amounts of label in DNA, higher than in RNA, could be only explained through direct reaction, since its slow turnover would require inordinately long exposure times to achieve the observed specific activities. The additional existence of different labeled bases, probably formed by the binding of formaldehyde and the "normal" bases not coinciding with any of the other bases. The thin layer chromatograius show a single spot, resolved in at least three peaks, none of which coincided with adenine, guanine nor thymine. The lack of label in these spots is incompatible with the "one-carbon" pathways hypothesis of label incorporation, since two "IC" units are needed for the synthesis of adenine and guanine and one for that of thymine. The presence of label in other different molecules strongly supports the adduct formation postulate, attributing to formaldehyde the main responsibility for the appearance of aspartame-methanol label in tissue components. The evidence presented, then, proves that a significant portion of the methanol carbon of aspartame finds its way into adducts of proteins and nucleic acids under the conditions tested, both in normal and cirrhotic rats. The results presented show that the carbon adducts of protein and DNA could have been generated only from formaldehyde derived from aspartame methanol, since all the other biochemical forms in which this carbon may be found could not produce adducts with protein and nucleic acids.
The doses of aspartame given to the rats in this experiment were high, higher at least than that any human may receive daily with normal consumption of the additive -in the range of 2-6 mg/kg-day (46)-, but were similar to those used in comparable tests on rodents in which no ill-effects were detected. These doses were in the same range as the adi for humans established for Canada and the EC (40 mg/kg-day) (46). The dose administered was also lower than that used for toxicity studies, which have shown that even at very high doses aspartame does not produce immediately appreciable harm (I 7). Most of these studies, however, refer to direct acute toxicity effects, which were not observed either in the rats used in the present study (except, perhaps, for softer droppings in those subjected to the chronic treatment with aspartame gavages).
The amount of label recovered in tissue components was quite high in all the groups, but especially in the NA rats. In them, the liver alone retained, for a long time, more than 2 % of the methanol carbon given in a single oral dose of aspartame, and the rest of the body stored an additional 2 % or more. These are indeed extremely high levels for adducts of formaldehyde, a substance responsible of chronic deleterious effects (33) that has also been considered carcinogenic (34,47). The repeated occurrence of claims that aspartame produces headache and other neurological and psychological secondary effects -more often than not challenged by careful analysis-(5,9,10,15,48) may eventually find at least a partial explanation in the permanence of the formaldehyde label, since formaldehyde intoxication can induce similar effects (49).
The cumulative effects derived from the incorporation of label in the chronic administration model suggests that regular intake of aspartame may result in the progressive accumulation of formaldehyde adducts. It may be further speculated that the formation of adducts can help to explain the chronic effects aspartame consumption may induce on sensitive tissues such as brain (6,9,19,50). In any case, the possible negative effects that the accumulation of formaldehyde adducts can induce is, obviously, long-term. The alteration of protein integrity and function may needs some time to induce substantial effects. The damage to nucleic acids, mainly to DNA may eventually induce cell death and/or mutations. The results presented suggest that the conversion of aspartame methanol into formaldehyde adducts in significant amounts in vivo should to be taken into account because of the widespread utilization of this sweetener. Further epidemiological and long-term studies are needed to determine the extent of the hazard that aspartame consumption poses for humans.
This research was carried out within a general study of artificial sweeteners' toxicity supported through the Bosch & Gimpera Foundation, Barcelona. Thanks are given to Robin Rycroft, from the Languages Advisory Service of the University of Barcelona, for revision of the manuscript.
Aspartame. Aspartame labeled ("C) in the methanol carbon was custom-prepared by Amersham (Amersham,
The product had a specific activity of 433 MBq/mmol, and a chromatographic purity >98%. The standard dose given orally to the rats was 4.5 Mbq per kg of rat weight, always supplementing unlabelled aspartame (Sigma, St Louis, MO USA) to give a specific activity of 55 Mbq/mmol.
Acute and chronic administration of aspartame to normal rats. Sixteen week-old healthy adult male Wistar rats, weighing initially 380-460 g, were used.
The rats were housed in collective cages in a controlled environment (21-22'C; 70-75 % relative humidity; lights on from 08:00 to 20:00), and were fed a standard chow pellet (B&K, Sant Vicent dels Horts, Spain) and tap water ad libitum.
Two groups of rats were selected. The first group NC (Normal- Chronic, N=5) received a daily oral gavage of 0.68 mmol per kg of rat weight (200 mg per kg) of a water suspension (2.5 mL/kg) of non-radioactive aspartame (Sigma). This treatment was continued for I 0 days. On day I 1, the rats were administered a gavage of 4.5 Mbq per kg of rat weight of labeled aspartame in 68 µmol of cold aspartame per kg, in the same volume of the standard gavage. The second group NA (Normal-Acute, N=l2) was given a single dose of 4.5 Mbq per kg of rat weight of labeled aspartame in 68 µmol of cold aspartame per kg of rat weight. Prior to the administration of the last (or only) dose, blood was extracted from the tail vein and used for the measurement of biochemical parameters using a Spotchem dry strip (panel I and 2) analysis system (Menarini, Milano, Italy).
The rats chronically treated (NC group) were killed by decapitation 6 hours after the administration of the labeled aspartame gavage. The rats in the NA group were killed by decapitation at 15 or 30 min and at 1, 2, 6 or 24 hours after the administration of the final labeled aspartame load. All animals were dissected, and samples of blood plasma (heparinized), liver, kidneys, brain, cornea, retina, hind leg striated muscle, epididymal fat pads and interscapular brown adipose tissue were cut, weighed (blotted when necessary), and frozen in liquid nitrogen. The samples were preserved at - 20'C until processed.
Tissue samples were homogenized in water: methanol (4: 1) in order to limit the losses of free methanol, using an all-glass Tenbroek homogenizer. Aliquots of the homogenates were immediately counted for radioactivity using a water-miscible scintillation cocktail (Ecolite, from ICN, Costa Mesa, CA USA. Plasma samples were counted directly after mixing with the scintillation cocktail. In all cases, two countings, 24-hours apart were performed. In all cases we obtained the same countings; there were no samples showing a significant loss of radioactivity (purportedly due to the eventual evaporation of methanol to the head space of the vial). Thus it was assumed that no significant amounts of labeled methanol were present in the final homogenates. Aliquots of the homogenates were precipitated with trifluoroacetic acid to remove the protein from supernatants, and the two fractions were then counted separately.
Acute and chronic administration of aspartame to liver-damaged rats Six week-old healthy adult male Wistar rats weighing initially 100-120 g were used. The rats were housed and fed under the same conditions described above for the controls. The rats were made cirrhotic by means of three i.p. injections per week of carbon tetrachloride diluted 1:1 with corn oil (36). The rats received 0.4 mL injections during the first 2 weeks, then 0.6 mL until week 6 and finally 0.8 mL until week 10, when the period of treatment was considered finished, when the rats weighed 340-380 g.
Two groups of liver-damaged rats were selected. The first group CC (Cirrhotic-Chronic, N=5) received a daily oral gavage of non-radioactive aspartame for 10 days, and on day I I they received 4.5 Mbq/kg of labeled aspartame as in the NC group. The second group CA (Cirrhotic-Acute, N=1 1) was given a single dose of 4.5 Mbq/kg of labeled aspartame in 68 µmol of cold aspartame per kg as in the NA group. Tail vein blood was sampled from these animals, and its plasma stored frozen; this was later used to measure biochemical parameters as in group NA.
The CC chronically treated rats were killed by decapitation -as in the control series- 6 hours after the administration of the labeled oral bolus of aspartame, and those in the CA group were killed at 15 or 30 min and at 1, 2, 6 or 24 hours after receiving the labeled aspartame load. Samples of blood plasma and tissues were weighed, frozen and stored at -20'C until processed. Some samples of liver were preserved in 4 % formaldehyde and later used for the preparation of stained tissue sections in order to determine the degree of hepatic alteration (37). Blood and tissue samples were processed as described for normal rats.
Statistical comparison between means was determined with standard two-way anova programs, as well as with the Student's t test.
Nucleic acids analysis. Two additional adult rats were treated as in group NC, but they received the gavage for only three days. The last gavage contained 37 Mbq of radioactive aspartame. After killing, blood plasma and liver samples were obtained and frozen. Liver tissue was used for the extraction and purification of total RNA and DNA using the Tripure (Boehringer Mannheim, Germany) isolation reagents system. These preparations yielded pure fractions of DNA, RNA and protein. Nucleic acids content was determined by uv light absorption at 260/280 nm (38), and protein with the Bradford method (39). The radioactivity of these fractions was measured and used for the estimation of their specific radioactivity. The pooled DNA samples of the two rats used were hydrolyzed with 88 % formic acid at 170'C in a sealed glass ampoule (40), and the corresponding constituting bases separated through thin layer chromatography on 0. I mm thick cellulose plates (5716 Merck, Darmstadt, Germany), run against standards of 14C-labeled adenosine, guanine and thymine (all from ICN, Costa Mesa, CA USA) containing their cold counterparts (from Sigma, St Louis, MO USA). The mobile phases used were isopropanol: 25% ammonium hydroxide (4:1 by volume) and butanol: acetic acid: water (4: 1:1 by volume) (4 1). Spot radioactivity was measured by exposure of the chromatograms with the Bio-Rad Molecular Imaging Screen-BI (Bio-Rad, Hercules, CA USA) for several days. The plates were later read with a Bio-Rad Molecular Imager System GS-525 two-dimensional array radioactivity counter; this instrument provided a printed "photographic plate" of the bidimensional distribution of radioactivity in the chromatogram. Labeled standards of DNA bases were used to determine whether the hydrolyzed sample presented any radioactivity in their spots. Cytosine was not included as standard since no carbon from IC pool participates in its structure through the whole process of pyrimidine synthesis.
The DNA digest from the liver of rats exposed to labeled aspartame was also analyzed through HPLC, using a Kontron (Milano, Italy) UPLC fitted on line with a diode array detector 440 (Kontron) and an eluate scintillation detector LB 507 A (Beckman, Fullerton CA USA). The instrument was run with the Data System 450-MT2/DAD (Kontron) software. We used a sex cationic interchange column (Kontron) (250x4 mm, 10 µm), maintained at 25ºC, and total flow was 0.8 mL/min. An isocratic gradient of 100 % 10 mM ammonium phosphate buffer pH 5.56 was used. The scintillation detector used a cocktail ultima-flo M (Packard, Meriden IL USA) with a mixture ratio of 3:1. A series of standards of adenine, thymine and guanine were run under the same conditions. In all cases the radioactivity in the fractions was recorded.
Protein analysis. The rats used for nucleic acid analysis provided enough plasma samples for protein analysis; plasma proteins were selected because they could not be contaminated with nucleic acids. The plasma proteins (0.100 mL aliquots) were precipitated with 10% trifluoroacetic acid. Aliquots of the precipitated proteins were then hydrolyzed for 48 h at 110°C in 6N HCl in Teflon-sealed tubes with occasional shaking (42). The digests were filtered to remove the black Maillard adducts (which retained part of the radioactivity). The amino acids in the digests were derivatized with dinitrofluorobenzene, and the DNP-amino acids were separated by bidimensional thin layer chromatography (43) on 0. 1 5 mm thick silicagel plates (Polygram Sil G/UV254, Mocherey-Nagel, Düren, Germany). The presence of label in amino acid spots was measured as in the case of nucleic acids using the Bio-Rad Molecular Imager. In separate runs, 14C_Iabelled methionine (NEN, Boston, MA< USA) diluted with cold methionine (Sigma) was added to rat plasma, digested, derivatized and processed as indicated above. Thus, the DNP- methionine spot was identified; in any case, the position of standard amino acids in the bidimensional chromatogram was known (43). The derivatization method used prevented the contamination of the plates by radioactive materials different from amino acids, since only the DNP-derivatized compounds were recovered.
An aliquot of 0.2 mL of blood serum albumin (Sigma) dissolved in water (100g/L) was incubated for 2 h at 37ºC with 0.02 mL of a labeled substrate preparation, containing I nmol and 5 kBq of 14C-labeled: a) aspartame, b) formaldehyde (Amersharn), c) formic acid (Sigma), or d) methanol (Amersham)@ The samples were then precipitated, washed with 10% trifluoroacetic acid and the precipitates counted for radioactivity. The protein exposed to formaldehyde retained a large proportion of the initial radioactivity added. In the cases of aspartame, formic acid and methanol, only background values were obtained in the washed protein precipitates, showing that none of these procedures resulted in stable label attachment to proteins. The samples of albumin exposed to formaldehyde label were processed in parallel to the sample of plasma (i.e. hydrolysis, derivatization and thin layer chromatography).
Table 1 shows the blood chemistry of the rats used. Aspartame administration, either chronic or acute (NC, NA groups), did not result in significant changes in plasma composition of the rats. In the cirrhotic rats, groups CC and CA, the plasma chemistry was deeply altered. The liver cytology (data not presented) together with altered transaminase levels and plasma chemistry showed that the CC and CA rats were affected by liver cirrhosis. The rats with cirrhosis showed lower urea, albumin and, especially, triacylglycerol levels than the controls. Aspartame administration resulted in no changes in plasma chemistry in normal rats.
Figure 1 shows the radioactivity found in several tissues of rats receiving a single oral dose of labeled aspartame. Liver, blood plasma and kidneys showed the higher radioactivity levels, in the range of 0. 1-0.4 % in each gram of fresh tissue of the dose administered. Since the dose given to each rat was 10 mg, of which a 10.5 % corresponded to methanol (i.e. I mg), 1/1000th of the dose given was just I µg, which means that 0. I% of the dose per gram of tissue was equivalent to 1 µgof methanol/ formic acid/ formaldehyde (= 31 mnol = I ppm). Liver, thus, contained between 1 and 3.7 ppm of label, while plasma and kidneys maintained very stable levels of about 2 ppm, following administration of a single dose. Chronic administration of aspartame (NC group) resulted in a higher yield of label after the last administration, as observed when comparing the data for 6 hours, ranging from 130-140 % of the value obtained in the single NA group. A fairly conservative estimate may indicate that the daily incorporation of aspartame carbon was in the range between 2 and 4 ppm for liver tissue, i.e. after 11 days the accumulation may be up to 30 ppm. In the cirrhotic rats, the pattern of label distribution was quite similar to that of healthy rats. In general, the amount of radioactivity in liver and kidney was lower, but higher in WAT than in normal liver rats.
The counting of radioactivity in plasma after acid precipitation of protein (which would set free formic acid and methanol, but not formaldehyde) gave a yield of less ffim 2 % of total label in the supernatant, i.e. practically all the radioactivity in the plasma at 6 hours was bound to protein. The same experiment done with liver gave a yield of 20-23 % of the label in the supernatant, the rest bound to protein and nucleic acids. The form of the time-course of label present in liver agrees with this finding, since there is a certain decay of label present in that organ with time from a peak at 60 min. This same pattern can be found in several other tissues (brown adipose tissue, muscle, brain and eye), but in the end, a significant part of the label can be assumed to be retained bound to protein.
The specific radioactivity of liver RNA, DNA and protein in the rats treated with very high specific activity labeled aspartame are presented in:
Table II despite considerable variability in the individual data, RNA showed lower specific activity than DNA and protein had the higher values per mg. The data are also expressed as a ratio of altered versus total structural unit (nucleotide / amino acid), i.e. units incorporating one of the labeled carbons derived from aspartame versus total nucleotides or amino acids. This ratio was obtained by dividing the specific activity found by that of the aspartame in the gavage. The ratios obtained show that the uniformity between protein and DNA was higher than when expressed per unit of weight. Cirrhotic rats showed high liver specific activities, in the same general range as the normal rats did. Roughly, the liver contained about one quarter of its label in "soluble" form, 2/3 in protein and less than 10% in nucleic acids, with a higher share in DNA than in RNA.
Figure 2 depicts the distribution of label in two thin layer chromatograins, the first showing the label distribution of DNA hydrolysates, from the rats receiving high specific activity aspartame, and the second, run under the same conditions, depicts the location of labeled adenine, guanine and thymine spots. In the DNA hydrolysate, the radioactivity present in the adenine, guanine and thymine spots was nil, since the label was present in another different and distinct spot, which was assumed to correspond to the adduct products of meffimol-derived carbon and DNA constitutive bases. The Rf values for the bases and the adduct were quite different: adduct 0.05/0.0 (first run/second run), guanine 0.10/0.22, adenine 0.40/0.43, thymine 0.57/0.49.
The separation through HPLC of the labeled fractions in the DNA hydrolysate resulted in three main peaks, eluting at 7.65, 11.94 and 12.86 min. Thymine eluted at 8.95 min, guanine at 9.42 min and adenine at 12.28 min under the same conditions.
Figure 3 shows the distribution of radioactivity in three thin layer chromatography plates. The first plate shows the label distribution obtained after processing the product of plasma protein hydrolysis from rats treated with high specific activity labeled aspartame. The second plate shows the results of an albumin sample exposed to labeled formaldehyde and ran in parallel with the other samples.
The third plate contains the spot of DNP-methionine. The Rf values for the radioactive spots were: in vivo labeled plasma protein 0.24/0.86 (first run/second run), in vitro labeled albumin: three spots, A 0.02 /0.0, B 0.38 / 0.0 and C 0.38 /0.88, DNP-methionine 0.44 / 0.51. The plates were considerably loaded with sample in order to obtain a minimal radioactivity recording. This resulted in long "tails" and blurred spots. In any case, there was a fair coincidence in one of the spots of in vitro labeled albumin (C) with that observed in the in vivo labeled plasma proteins. The methionine spot was quite different from this one. In addition, the radioactive spot of exposed rat protein (and those of formaldehyde-labeled plasma proteins) were not coincident with any of the standard protein amino acids.
TABLE 1 <Back
Plasma parameters in rats acutely or chronically treated with oral aspartame
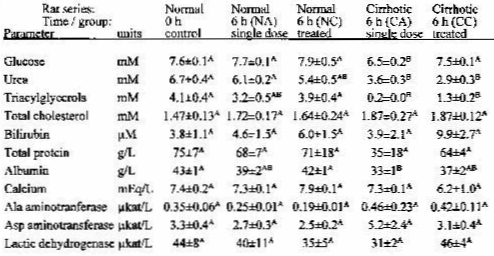
The data correspond to the mean +/- scm of 4 animals per group. Statistical significance of the difference between groups: all groups with different raised letters are different (p<0.05, Student's test)
Figure 1 <Back
Specific activity of liver RNA, DNA, and protein in rats receiving high specific activity gavage of labeled protein
Table II <Back
The data are the mean of two different an same kind present in the liver tissue of animals.
FIGURE 2 <Back
The specific activity in dpm/mg refers to mg of RNA, DNA or protein. The expression "unit ratio x 106" represents the number of units (nucleotides, amino acids) incorporating carbon units derived from oral aspartame per million of the

Distribution of radioactivity in the nucleotides resulting from the hydrolysis of DNA of rats treated with labeled aspartame.
Thin layer chromatograms on cellolose plates showing: the result of the hydrolysis of DNA from rats receiving high specific activity gavage of aspartame (chromatogramin the left; 25Hq. 4 days exposure) and standards for adenine A, guanine G and thymine T (chromatogram in the right, 220 Bq each, 1 day exposure.
FIGURE 3 <Back

Distribution of radioactivity in the DNP-amino acids resulting from the hydrolysis of plasma proteins of rats treated with labeled aspartame. Bidimensional thin layer chromatograms on silicagel plates showing: the spot obtained from hydrolyzed plasma proteins of rats treated with labeled aspartame after hydrolysis and derivatization (chromatogram in the left, total about 50 Bq, 4 days exposure), the spots obtained exposing in vitro albumin to labeled formaldehyde, after hydrolysis and derivatization (chromatogram in the center, total about 110 Bq, 4 days exposure) and the spot for labeled DNP-methionine (chromatogram in the left, 180 Bq, 1 day exposure).
1. S.L. BURGERT, D.W. ANDERSEN, K.D. STEGINK, H. TAKEUCHI and H.P. SCHEDL. Metabolism 40 612-618 (1991)
2. N.M. HOOPER, R.J. HESP and S. TICKER. Biochem. J. 298 635-639 (1994).
3. W.E. LIPTON, Y.N. LI, M.K. YOUNOSZAI and L.D. STEGINK. Metabolism 40 1337-1345 (1991).
4. M. KOIVUSALO. International Encyclopedia of Pharmcology and Therapeutics. J. Tremoličres (Ed.) Vol. 2 Sect. 20, 564-605. Pergamon Press, New York, (1970).
S. S.K. VAN DEN EEDEN, T.D. KOEPSELL, W.T. LONGSTRETH, G. VANBELLE, J.R. DALING and B. MCKNIGHT. Neurology 44 1787-1793 (1994).
6. R.J. V,7URTh4AN. New Eng. J. Med. 309 429-430 (1983).
7. M.M. GARRIGA, C. BERKEBILE and D.D. METCALFE. J. Allerg. Clin.Immunol. 87 821-827 (1991).
8. R. GEHA, C.E. BUCKLEY, P. GREENBERGER, R. PATTERSON, S. POLMAR, A. SAXON, A. ROHR, W. YANG and M. DROUIN. Clin. Experiment. Allergy 24 175
(1994).
9. A. KOESTNER. Aspartame, Physiology and Biochemistry. L.D. Stegink and L.J.Filer (Eds.), 447- 45 7. Marcel Dekker, New York (I 984).
10. H.J. ROBERTS. Clin. Res. 38 A798 (1990).
11. S. SARAVIS, R. SCHACHAR, S. ZLOTKIN, L.A. LEIT'ER and G.H. ANDERSON.
Pediatrics 86 75-83 (1990).
12. H.A. TILSON, J.S. HONG and T.J. SOBOTKA. Neurotoxicol. Teratol. 1 27-35 (1991).
13. L. TOLLEFSON and R.J. BARNARD. J. Arn.Diet. Assn. 92 598-601 (1992).
14. P.A. SPIERS, D.L. SCHOMER, R.J. WURTMAN, L. SABOUNJIAN, A. REINER, D.MYERS and J. WURTMAN. J. Clin. Exp. Neuropsychol. 15 407 1993.
15. R.G. WALTON, R. HUDAK and R.J. GREENWAITE. Biol. Psychiat. 34 13-17 (1993).
16. A. LAJTHA, M.A. REILLY. and D.S. DUNLOP. J. Nutr. Biochern. 5 266-283 (1994).
17. L.D. STEGINK. Am. J. Clin. Nutr. 46 204-215 (1987).
18. K.P. PORIKOS and T.B, VAN ITALLIE. Aspartame, Physiology and Biochemistry. L.D.Stegink and L.J. Filer (Eds.), 273-286. Marcel Dekker, New York (1984).
19. D.A. BOOTH. Developments in Sweeteners. T.H. Grenby (Ed.), Vol.3 287-316. Elsevier, London(1987).
20. T.R. TEEPHLY and K.E. MCMARTIN. Aspartame: Physiology and Biochemistry. L.D.SteginkandL.J.Filer(Eds.),111-140.MarceiDekker,NewYork(1984)..
21. J.T. EELLS, C. RAJANI, T.G. MURRAY, M. LEWANDOWSKI and J.M. BURKE. invest. Ophthalmol. Vis. Sci. 31 143 (1990).
22. L.J. FREDERICK, P.A., SCHULTE and A. APOL. Am. Ind. Hyg. Assoc. 45 51-55 (1984).
23. K.E. MCMARTIN, A.B. MAKAR, G. MARTIN-AMAT, M. PALESE and T.R. TEPHLY. Biochem. Med. 13 319-333 (1975).
24. J.T.EELLS, K.A. BLACK, C.E. TEDFORD and T.R. TEPHLY. J. Pharmacol. Exp. Ther. 227 349-353 (1983).
25. K.A. BLACK, T.J. EELLS, P.E. NOKES, C.A. HAWTREY and T.R. TEPHLY. Proc. Nat. Acad. Sci. USA 82 3854-3858 (1985).
26. C.S. LIEBER and L.M. DE CARLI. Science 162 917-918 (1968).
27. C.D. BENTON and F.P. CALHOUN. Trans. Am. Acad. Ophthalmol. 56 875-
883 (1952).
28. J.T.EELLS. J. Pharrnaeol. Exp. Ther. 257 56-63 (1991).
29. N.H RASKIN and L. SOKOLOFF. J. Neurochem. 19 273-282 (1972).
30. P. JULIŔ,J. FARRÉS and X. PARÉS. Biochem. J. 213 547-550 (1983).
31. R.H. HASCHEMEYER and A.E.V. HASCHEMEYER. Proteins. 11-30. John Wiley & Sons, New York (1973).
32. D.E. METZLER. Biochemistry, 90-107. Academic Press, New York.(1977).
33. H.D. HECK, M. CASANOVA and T.B. STARR. Crit. Rev. Toxicol. 20 397-426 (1990).
34. A. BLAIR, R. SARACCI, P.A. STEWART, R.B. HAYES and C. SHY. Scad. J. Work Environ. Hlth. 16 381-393 (1990).
35. J.A. OPPERMANN, E. MULDOON and R.E. RANNEY. J. Nutr. 103 1454-1459 (1973).
36. F. BLONDÉ-CYNOBER, F. PLASSART, C. REY, C. COUDRAY-LUCAS, N. MOUKARBEL, R. POUPON, J. GIBONDEAU and L. CYNOBER. Ann. Nutr. Metab. 38 238-248 @1994).
37. K.G. ISHAK, H.J. ZIMMERMAN and B. RAY. Alcoholism 15 45-66 (1991).
38. S.R. GALLAGHER. Current Protocols in Molecular Biology. Vol. 3 A.3D.1-A.3D.8. Wiley, Boston (1996).
39. MM. BRADFORD. Anal.Biochem. 72 248-254 (1976).
40. A. BENDICH. Meth. Enzymol. 3 715-723 (1956).
41. A.H. VAN GENNIP, N.G.G.M. ABELING and D. DE KORTE. Handbook of TLC. J. Sherma and B. Fried (Eds.). Vol. 55 863- 906. Chromatographic Science Series (1990).
42. G. ALLEN. Laboratory Techniques in Biochemistry and Molecular Biology. R.H. Burdan and P.W. van Knippenberg (Eds.)Vol. 9 40-42. Elsevier, Amsterdam (1989).
43. P. ROCA, A. PALOU and M. ALEMANY. J. Biochem. Biophys. Meth. 8 63-67 (1983).
44. F. MION, A. GÉLOEN, E. AGOSTO and Y. MINAIRE. Hepatology 23 582-588 (1996).
45. C. ADÁN, A. ARDÉVOL, X. REMFSAR, M. ALEMANY and J.A. FERNÁNDFZ- ÓPEZ Arch. Int. Physiol. Biochim. Biophys. 102 55-59 (1994).
46. H.H. BUTCHKO and F.N. KOTSONIS. Nutr. Toxicol. 11 235-250 (1994).
47. T.D. STERLING and J.J. WEINHAM. Exp. Pathol. 37 128-132 (1989).
48. J.W. OLNEY, N.B. FARBER, E. SPITZNAGEL and L.N. ROBINS. J. Neuropathol. Exp. Neurol. 55 1115- 1123 (1996).
49. L. BRETHERICK (Ed.) Hazards in the chemical laboratory. 3rd. ed., 339. Royal Society of Chemistry, London (I 98 1).
51. T.J. MAHER and R.J. WURTMAN. Hlth. Perspect. 75 53-57 (1987).